- Inicio
- freestyle libre 2
- Abbott's FreeStyle® Libre 2 iCGM Cleared in U.S. for Adults and Children with Diabetes, Achieving Highest Level of Accuracy and Performance Standards - Jun 15, 2020
Abbott's FreeStyle® Libre 2 iCGM Cleared in U.S. for Adults and Children with Diabetes, Achieving Highest Level of Accuracy and Performance Standards - Jun 15, 2020
5 (460) · € 5.50 · En stock
Abbott (NYSE: ABT), the worldwide leader in continuous glucose monitoring (CGM), announced today the U.S. Food and Drug Administration (FDA) cleared its next-generation FreeStyle® Libre 2
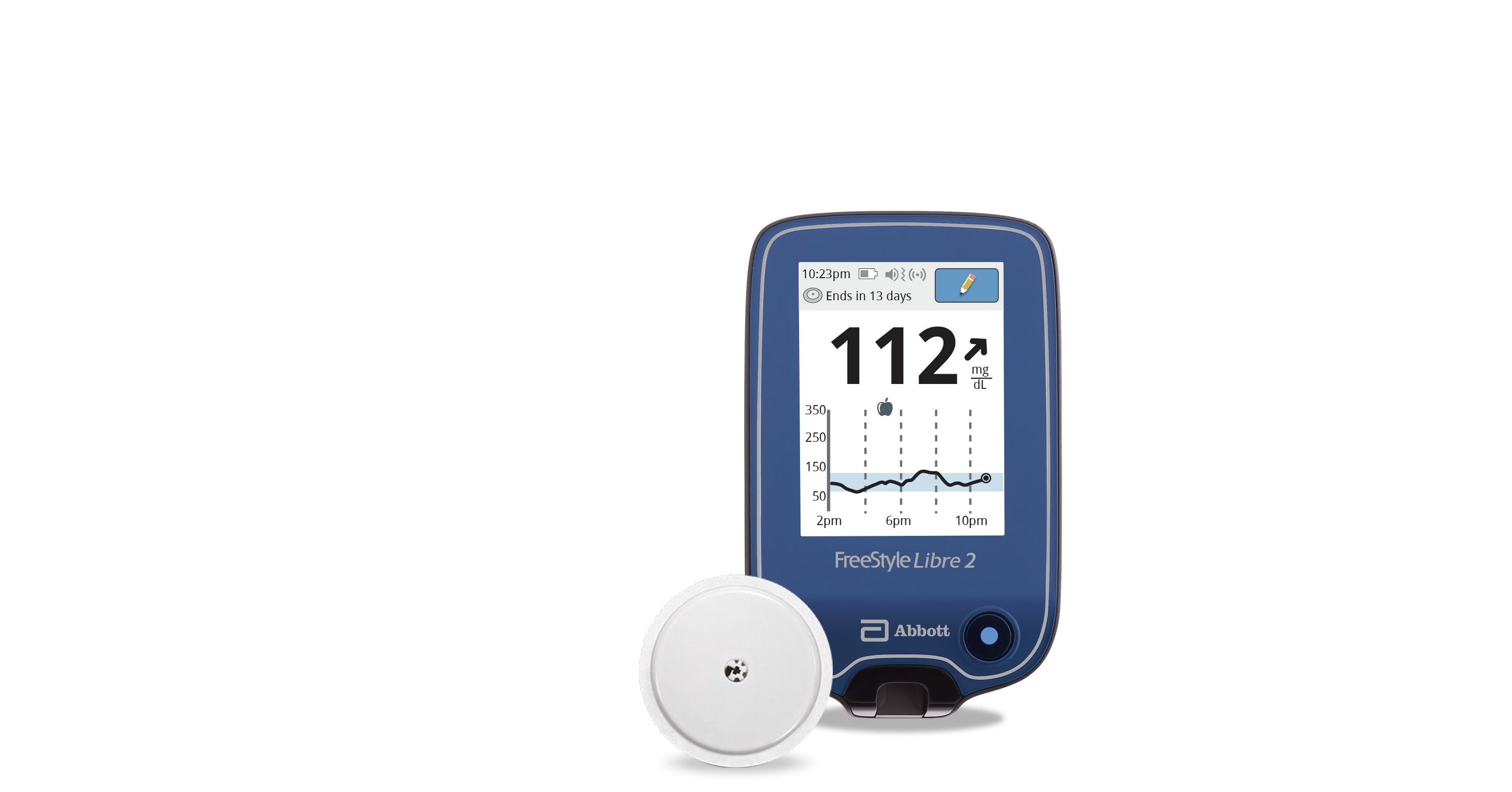
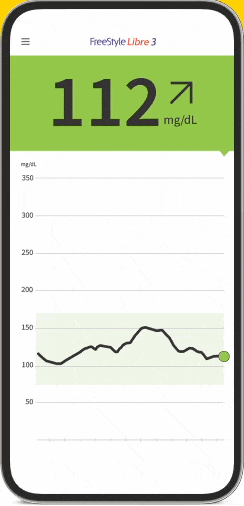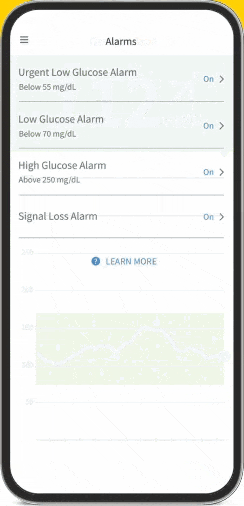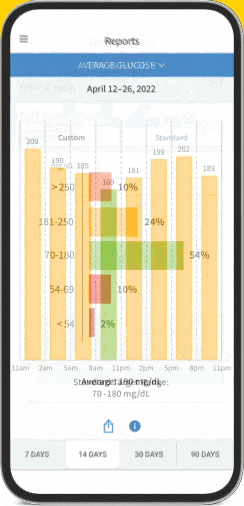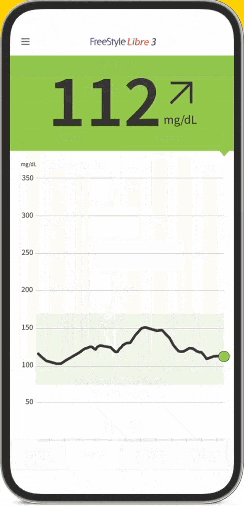
FreeStyle Libre 2 System (CGM)

CGM accuracy: Contrasting CE marking with the governmental controls of the USA (FDA) and Australia (TGA): A narrative review - Pemberton - 2023 - Diabetes, Obesity and Metabolism - Wiley Online Library

FreeStyle Libre 2 Continuous Glucose Monitor

FreeStyle Libre 2 System (CGM)

Abbott Releases the FreeStyle Libre 2 iCGM - Children with Diabetes
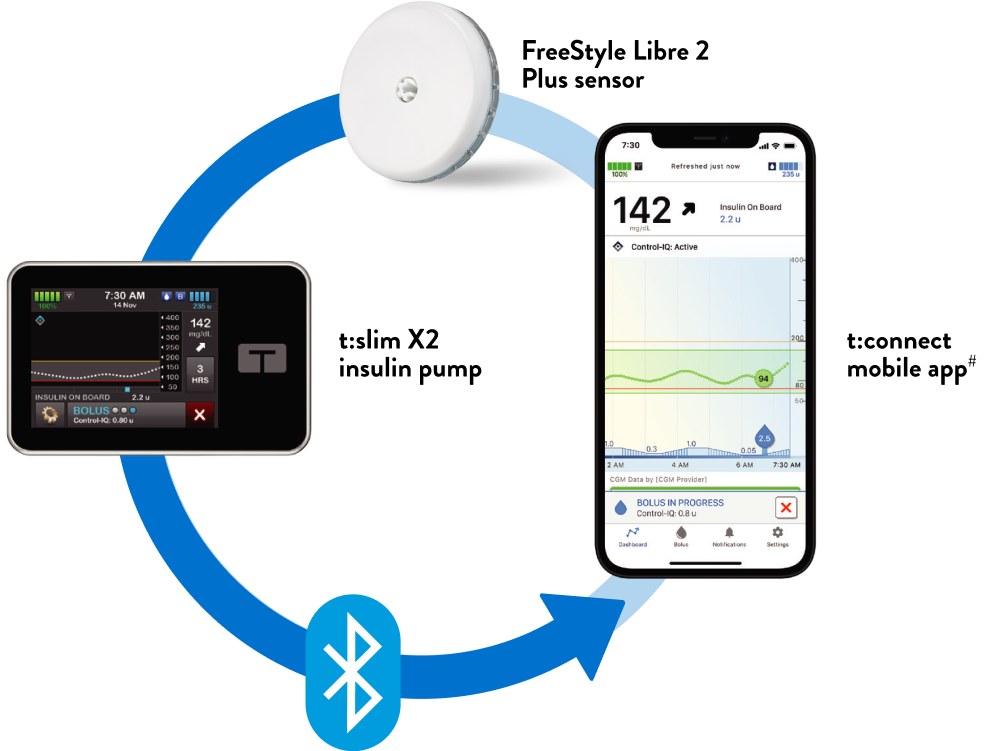
U.S. FDA Clears Abbott's FreeStyle Libre® 2 and FreeStyle Libre® 3 Sensors for Integration with Automated Insulin Delivery Systems

Landscape of Continuous Glucose Monitoring (CGM) and Integrated CGM: Accuracy Considerations
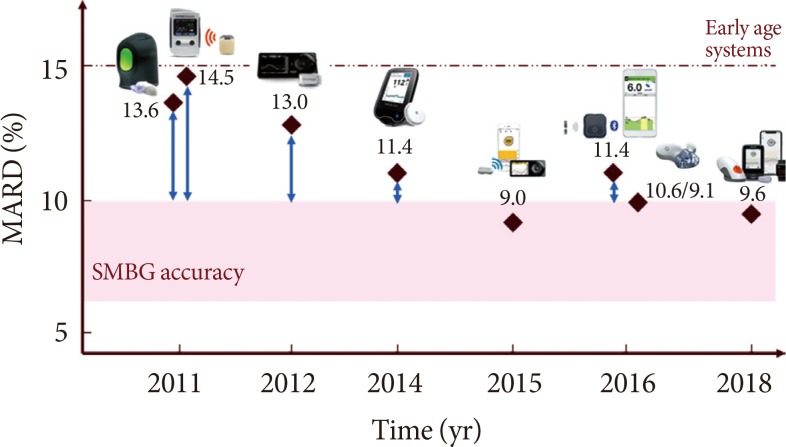
Continuous Glucose Monitoring Sensors for Diabetes Management: A Review of Technologies and Applications
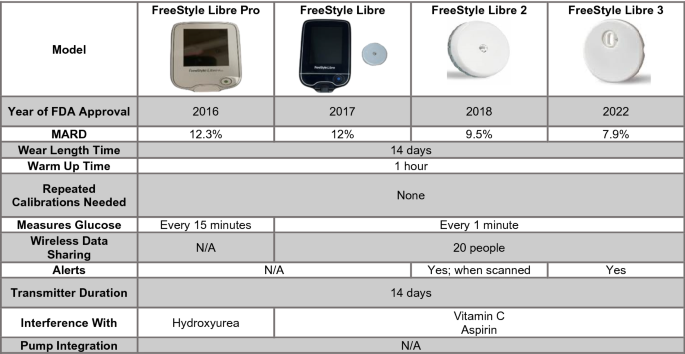
Emerging Diabetes Technologies: Continuous Glucose Monitors/Artificial Pancreases

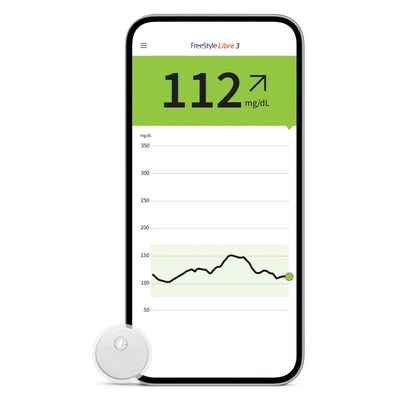
FreeStyle Libre 3: World's Smallest Sensor