- Inicio
- delta q
- thermodynamics - Confused why delta ∆Q and dQ don't make sense for heat Q - Chemistry Stack Exchange
thermodynamics - Confused why delta ∆Q and dQ don't make sense for heat Q - Chemistry Stack Exchange
4.9 (653) · € 22.50 · En stock
In my chemistry teacher's notes, some notations concerning the heat $Q$ are marked as inappropriate. $Q$: yes d$Q$: no $\delta Q$: yes $\Delta Q$: no In the second bullet in the screenshot below

Heat Transfer Basics – Part Zero

Tackling a Century Mystery: Entropy
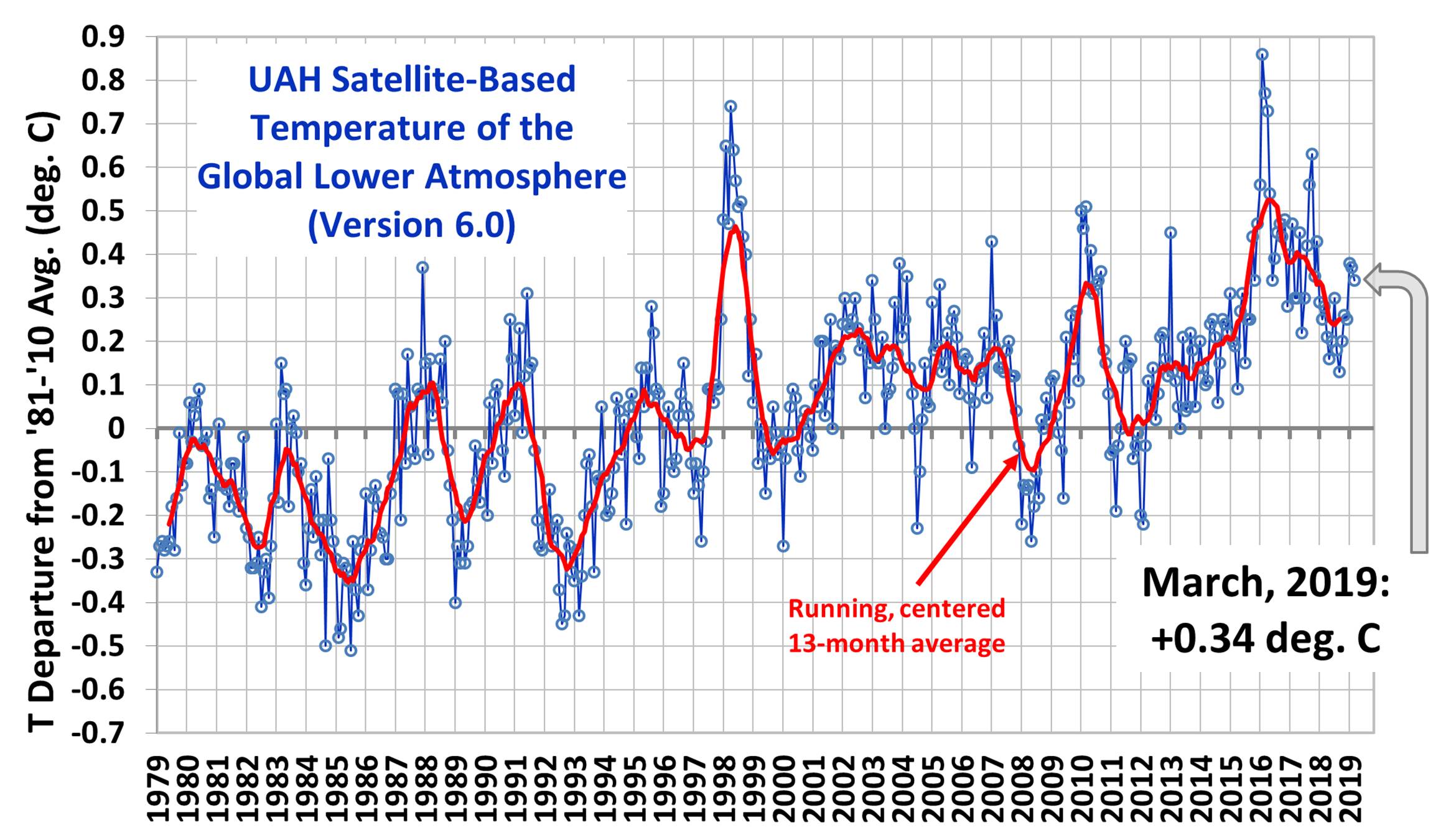
UAH Global Temperature Update for March, 2019: +0.34 deg. C. - Roy Spencer, PhD.
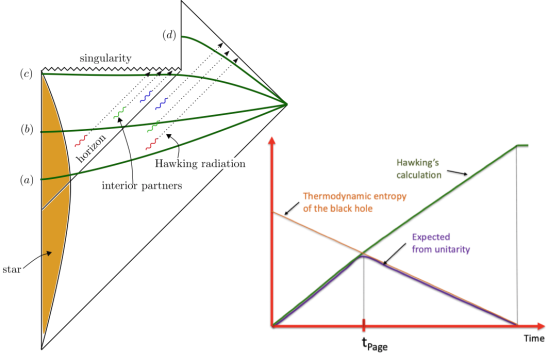
Ro's blog Musings of a nascent physicist

PDF) Fundamentals of Chemical Engineering Thermodynamics
Thermodynamics says, entropy increases with temperature. But can't we say vice versa, increasing entropy increases the temperature? I mean, entropy increases through every act, then doesn't that mean the earth is heating

Quantum Theory of Solvent Effects and Chemical Rea - Survival
While calculating change in entropy of surrounding, which type of heat is taken, reversible or irreversible? - Quora
How is the Second Law of Thermodynamics equation different from other physics equations? - Quora
Conservation of Energy Brilliant Math & Science Wiki

105 questions with answers in STATISTICAL PHYSICS
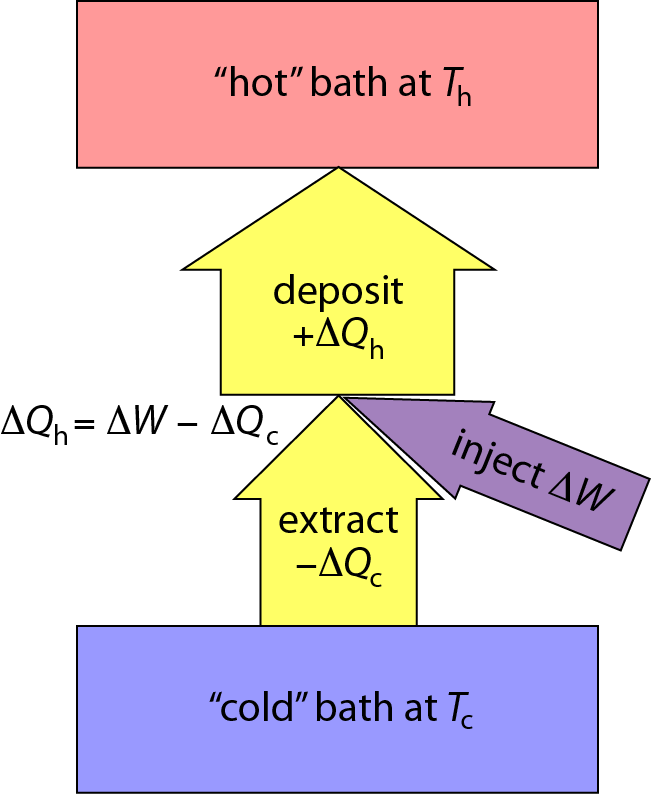
Heat Pumps Work Miracles

Thermodynamic & Chemical Equil PDF, PDF, Heat












